The negative effects of hydrogen on the atmosphere
A growing number of headlines increasingly warn about the potential risks of hydrogen as a substitute for fossil fuels. Most of them claim that so-called green hydrogen might not be as environmentally friendly as we are led to believe. But what is the truth behind this? Is the fossil fuel lobby behind these news because they fear the loss of their power? Could the treatment be worse than the disease?
First of all, many studies have been conducted for decades on the effects of hydrogen in the Earth’s atmosphere. These research studies, which will be discussed further below, highlight the fact that hydrogen emissions to the atmosphere are not good news for our planet. The scientists have already pointed out the potential risks associated with hydrogen emissions, of which we need to be aware if we wish to shift our economy towards hydrogen. Replacing a large part of fossil fuels with hydrogen, as is being proposed in the Roadmaps of different countries, could easily double or triple the current levels and therefore its negative effect on the atmosphere.
Studies show potential risks due to the unavoidable leakage that could arise from its normal use. Once again, one of the major drawbacks with hydrogen, its high leakage capacity owing to its small size and low density becomes our disadvantage.
It is also important to highlight that, in the long term, over a hundred year period, one tonne of hydrogen in the atmosphere will indirectly heat the Earth about 11 times more than one tonne of CO2.
What is the effect of hydrogen into the atmosphere?
As a light gas, when hydrogen is emitted into the air, it quickly rises into the atmosphere, thereby rapidly increasing the number of molecules in it.
There are two adverse effects that have been identified as potentially caused by this increase of hydrogen emitted into the atmosphere:
- On one hand, it would reduce the ozone in the stratosphere through the produced humidity.
- On the other hand, the climate change contribution through an increase of methane and its effect on the tropospheric ozone production.
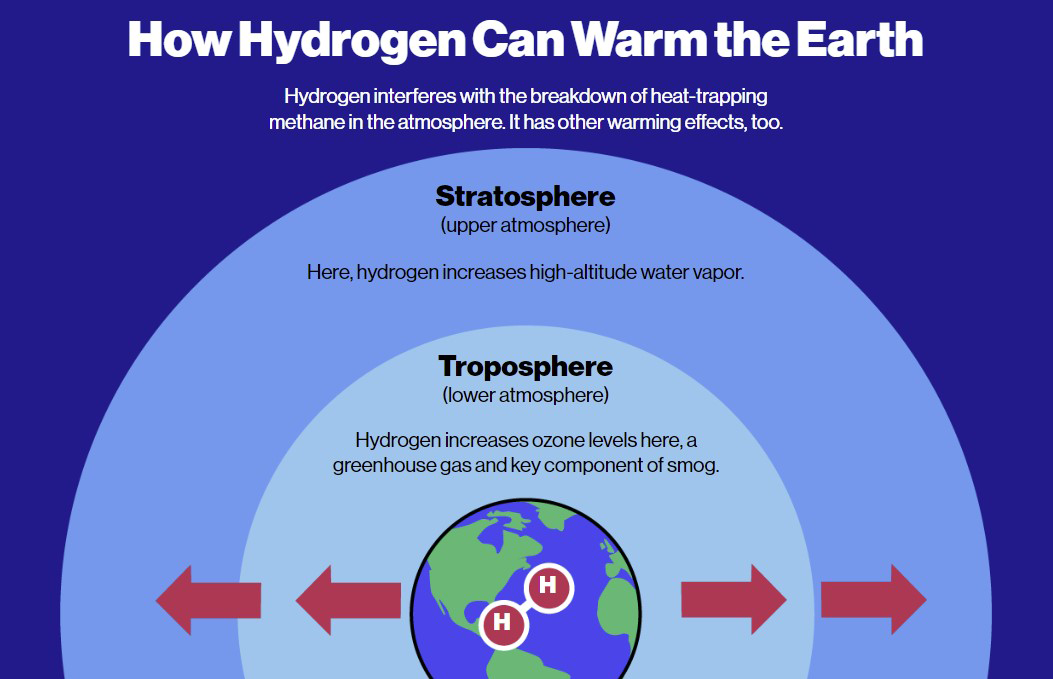
These two issues are the consequences of the main atmospheric hydrogen removal process:
OH + H2 → H2O + H
Although this reaction does remove hydrogen from the surrounding atmosphere, its side effect is to produce water vapour (H2O). While a production at lower atmospheric levels has few adverse consequences, excessive production of water vapour at upper atmospheric levels can be harmful. This water vapour in the upper atmosphere could cool the stratosphere, which may change the distribution of polar stratospheric clouds that are important in the creation of ozone holes and, therefore, may delay the recovery of the ozone layer. At this altitude, the growth of water molecules causes an enlargement of the hole already created at the poles, leading to a further loss of the ozone layer.
Besides, hydrogen is also mentioned as an indirect greenhouse gas. By reacting with OH radicals, hydrogen has an additional side effect that reduces the availability of OH radicals, which potentially impacts on the accumulation of greenhouse gases. In the lower atmosphere, hydrogen can accelerate the accumulation of greenhouse gases such as methane and thereby contributes to climate change by reacting with the same tropospheric oxidants that ” clean up” methane emissions and interferes in ozone growth.
Methane and ozone are among the most important greenhouse gases alongside carbon dioxide and NOx. In fact, methane is an astonishingly powerful greenhouse gas, responsible for 80 times more greenhouse warming than the CO2 equivalent over its first 20 years. However, atmospheric radicals scavenge the atmosphere quickly, whereas CO2 remains for thousands of years, so CO2 is worse long-term. When hydrogen is in the atmosphere, those radicals react with it, so there are fewer “cleaning” agents for other elements, increasing methane concentrations directly, and it will remain longer in the atmosphere.
Hydrogen has not been as well studied nor has it been given as much attention by the atmospheric research community as other gases. Mostly because, between its release into the atmosphere and disposal time, hydrogen causes no direct harm to human health or target ecosystems, and is therefore considered neither an atmospheric pollutant nor to have a significant ground-level effect as other gases.
While all these concerns remain uncertain and result of theoretical studies, we must be aware of the risks of hydrogen, minimising leakage as much as possible and controlling hydrogen levels in the atmosphere.
While there is a risk that more hydrogen use could damage the ozone layer resulting in climate change, the greenhouse effect from an economy based on hydrogen is likely to be reduced. In the end, hydrogen will reduce the use of methane and other gases responsible of the global warming, so there will be less emissions in the atmosphere and the lack of OH will be less relevant.
Therefore, the environmental risks of the hydrogen economy are supposed to be minor compared to its environmental benefits. Understanding pros and cons of each technology will help us choosing the best option for our planet which should be our society’s responsibility towards future generations.
This situation is quite unique as society has the opportunity of understanding the potential environmental impact far before the growth of the hydrogen economy.
What would have happened if we had known 100 years ago about the negative effect CO2 and Methane would have?
A new opportunity is now available for hydrogen
REFERENCES
Forster, P. M. de F., Shine, K.P., 2002. Assessing the climate impact of trends in stratospheric water vapor. Geophysical Research Letters 29, 10-1 – 10-4.