Hydrogen storage methods
How is hydrogen stored?
The following article reviews the different ways in which hydrogen can be stored and its current development status. Hydrogen will not be produced on-site and at the time of its consumption for many purposes. Instead, hydrogen can be produced at a central site and stored for further end-use distribution. Therefore, storage and/or transport systems are needed throughout the renewable hydrogen chain.
The most suitable storage vessel will be determined by the use of this storage, the volume to be stored, the length of storage, the required discharge rate, the geographical availability of different options and whether the storage is small-scale or large-scale. For large-scale storage, the energy density issue and filling time are not constraints for stationary applications, whereas for small-scale storage, the energy density issue and charging times are crucial for mobile applications. The purity of the hydrogen is an additional important factor, as a high purity of hydrogen is of utmost importance for its use in fuel cells, whilst for burning purposes, purity will not be a critical point.
Hydrogen has a high energy per unit mass content of 120.1 MJ/kg. However, its low density at environment temperature yields an extremely low energy density (0.01 MJ/L). As a result, larger volumes of hydrogen must be pumped to meet the same energy demands as other fuels. A volume of 11.2 Nm³ (the volume of the boot of a large utility or commercial vehicle) is needed to store just 1 kg of hydrogen, which is the amount required to travel approximately 100 km. Thus, for hydrogen storage to be economically viable, its storage density must be increased.
Hydrogen storage can be divided according to whether it is based on physical or material storage (see Figure 1). Under physical storage, it is stored as a gas or liquid as a pure molecular compound with no significant physical or chemical bonding to other materials. First, we start by analysing these storage methods, which are currently employed on a large scale.
Compression of hydrogen in its gaseous state can be accomplished by storing it in tanks or vessels, for small storage volumes, and geological storage, for large amounts.
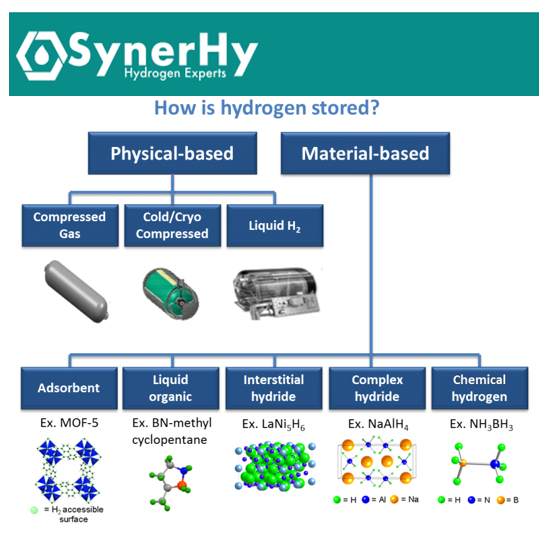
Figure 1. Categories of hydrogen storage methods
There are 4 types of high-pressure cylinders for tank storage. Type I are pressure vessels of metal material. H₂ for industrial gas is stored in Type I tanks, which have pressures between 150 and 300 bar. These are currently the most widely used and cheapest high-pressure tanks, but they are not suitable for use on vehicles due to their heavy weight. Type II pressure vessels are made of a thick metal ring liner wrapped with a carbon fibre or glass fibre material. They are used as high-pressure tanks in hydro generators, as they can withstand up to 1000 bar.
Type III tanks are pressure vessels consisting of an internal metal liner, to prevent hydrogen leakage by diffusion, fully wrapped with composites, which can withstand the mechanical stress. By removing thick metal walls and increasing composites, these vessels are less weighty than Types I and II. Type IV tanks, shown in Figure 2, are pressure vessels made of a high-density polymer liner, which acts as a gas diffusion barrier, fully enclosed with a carbon fibre compound. These tanks are equipped with metal valves for hydrogen refuelling and supply. They can withstand pressures of up to 700 bar. Type III and IV vessels are designed for portable applications, for which weight savings are essential, where pressures between 350 and 700 bar are required. However, these vessels are much more expensive due to the use of carbon fibre.
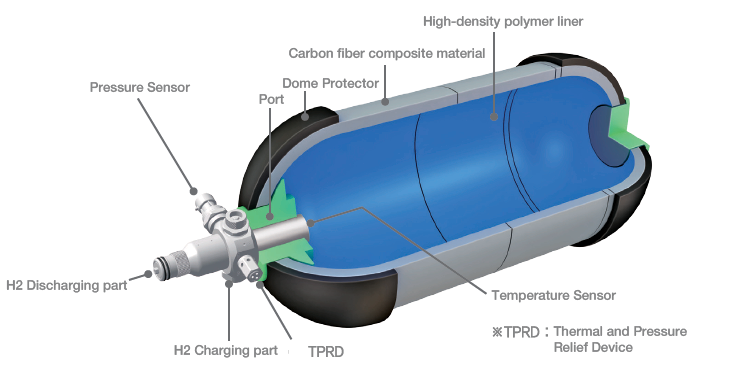
Figure 2. High pressure tank type IV
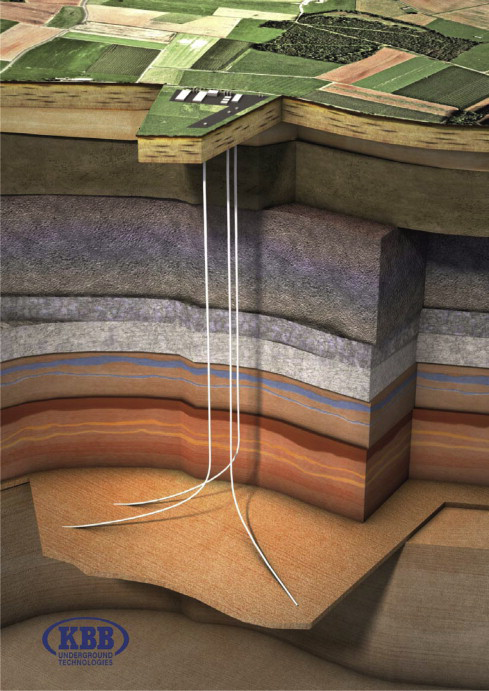
Figure 3. Geological storage
Salt caves, depleted natural gas and oil fields which are currently used to store natural gas, could be used for storing hydrogen. These underground storage possibilities allow for large-scale, long-term storage.
The United States currently has the largest salt cave hydrogen storage system in operation; it can store about 30 days of hydrogen production (between 10 and 20 thousand tonnes).
Oil and gas fields are usually larger than salt caves, but they are also more permeable and contain pollutants that would require removal before using the hydrogen in fuel cells. Water aquifers are the least mature among all options, and their suitability is still being discussed.
Besides compression, the density of pure hydrogen can also be increased through liquefaction (condensation), which converts hydrogen gas into liquid hydrogen (LH₂). Liquefaction is the process of converting a gas to a liquid by modifying its pressure and temperature conditions. Cryogenic temperatures are required for this process since the boiling point of hydrogen is -252.8 °C at one atmosphere of pressure. Liquefaction process employs a combination of compressors, heat exchangers and expansion valves to achieve the necessary cooling.
The simplest liquefaction process is the Linde cycle or Joule-Thompson expansion cycle. During this process, the gas first undergoes isothermal compression at environment temperature, followed by cooling at a constant pressure in a heat exchanger, and then finally an isenthalpic expansion. During the last stage, some of the gas liquefies and the remainder is re-circulated through the heat exchanger before flowing back to the compressor to complete the cycle.
The Linde cycle works on gases, for example nitrogen, which cool as they expand at environment temperature. However, hydrogen heats up while expanding at environment temperature. To cool hydrogen as it expands, its temperature must be lower than its reversal temperature, -95 °F. In order to achieve this temperature, the gaseous hydrogen is pre-cooled by evaporating liquid nitrogen to a temperature below -319 °F before the first expansion of the valve.
Once the hydrogen has been liquefied, it is essential that it can be stored in such a way as to minimise evaporation. This evaporation of liquid hydrogen is not only a loss of the energy invested in liquefying the hydrogen, but also a loss of hydrogen in the long term, as the evaporated gas must be vented due to the pressure accumulated inside the storage vessel. This loss of stored hydrogen over time is known as boil-off and is reported as the percentage of stored hydrogen lost per day: the boil-off rate. The heat transference from the surroundings to the stored liquid hydrogen and thus the boil-off rate is reduced by minimising the surface-to-volume ratio of the vessels by making them spherical, as shown in Figure 4.

Figure 4. Spherical LH₂ storage tank
LH₂ vessels are usually double walled with a gap between them. This gap minimises heat transfer by conduction and convection. The space between the walls of the vessel may contain additional materials to prevent heat transfer by radiation. Owing to the high degree of insulation and the low surface-to-volume ratio, boil-off rates are very low in larger spherical tanks, usually below 0.1 % per day.
It is worth highlighting the fact that in Germany’s network of over 100 hydrogen plants (HRS), both the transport of hydrogen in trucks and the storage of hydrogen is done in its liquid form. Cryocompressors are used to convert it into gas and compress it to almost 1000 bar for cascade refuelling, as we saw in a previous blog article (see video below).
Liquid hydrogen is the technology that achieves the highest storage energy density (8.4 MJ/L, twice as much as with 700 bar compression systems). A disadvantage of this method is the long loading and unloading times. It is suitable for storing and transporting high amounts of hydrogen. Nevertheless, if the storage period is going to be long, it is recommended to use other options, since this method requires a much higher energy input than that needed for high-pressure gaseous hydrogen compression, as can be seen in Table 1.
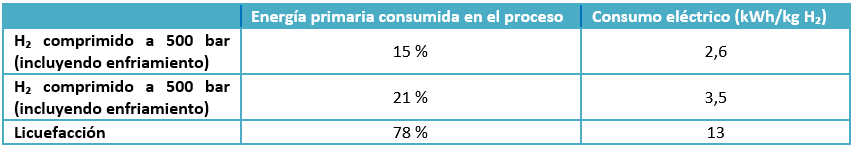
Table 1. Comparison of energetic intake between CH₂ and LH₂
Cryo-compressed hydrogen storage (CcH2) is a hybrid method between CH₂ and LH₂. This method stores H₂ at cryogenic temperatures in a vessel that can be pressurised (nominally at 250-350 bar), unlike current cryogenic vessels that store LH₂. Cryo-compressed tanks can store liquid hydrogen, supercritical cryogenic hydrogen or two-phase state hydrogen (saturated liquid and vapour). The storage of liquid hydrogen in isolated pressure vessels overcomes many of the weaknesses of CH₂ or LH₂ tanks and may even unlock new opportunities. However, this storage technology is not yet fully developed nowadays.
At this point we will discuss material-based storage methods. They are classified into storage by adsorption on the surface of solids, supported by fairly weak physical van der Waals bonds, or storage by absorption of atomic hydrogen within solids.
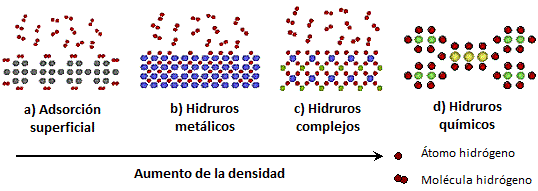
Figure 5. Several ways of storing hydrogen in materials
Hydrogen can be adsorbed in a reversible way on the surface of a porous solid, both in molecular and atomic states. Hydrogen storage by adsorption exploits the physical van der Waals bonding between molecular hydrogen and a material with a high specific surface area. Given the weakness of the van der Waals bond, low temperatures and high pressures are usually required to achieve significant hydrogen storage densities by adsorption. Heat management is one of the most important challenges for this technology, where liquid nitrogen is the most common refrigerant used in the process. Pressure is usually between 10 and 100 bar, but it varies according to the adsorbent and the intended use. Higher pressures are not an advantage beyond a certain threshold, because the presence of the adsorbent may no longer improve the hydrogen storage capacity versus the pressurised gas being stored in the same vessel, due to the space required by the adsorbent.
There have been several proposals for adsorbents to store hydrogen, such as porous carbon-based materials, metal-organic structures, zeolites, etc. Contrary to CH₂ or LH₂ storage, there is limited experience in applying this technology and most of the developed adsorption-based vessels have only been designed on bench scale.
Storage by absorption can be accomplished by means of liquid organic hydrogen carriers, metal hydrides, complex hydrides or chemical hydrides.
Liquid organic hydrogen carriers (LOHCs) are organic compounds which can absorb and release hydrogen through reversible hydrogenation and dehydrogenation processes. Hydrogenation is a chemical reaction where hydrogen is added to another compound as a result of the chemical reaction. Since both the hydrogenated and dehydrogenated forms of LOHCs are liquid at environment conditions, there is an advantage as there is no need to produce, capture or store CO₂ or N₂, as well as the hydrogen obtained after dehydrogenation is relatively pure. Therefore, LOHCs allow hydrogen storage at atmospheric pressure and temperature.
The process, illustrated in Figure 6, comprises the addition of hydrogen to the carrier substance at source, while it is cooled and slightly pressurised, thereby turning it into a different substance. This new substance is transported to the destination (by truck or ship etc.). On site to be used, the substance is heated while slightly decreasing the pressure. As a result, the hydrogen is released and returned to the initial substance, which can be transported to the source to start the process all over again. This technology can exploit existing infrastructure, as standard tanks can be used at harbours and industrial areas.
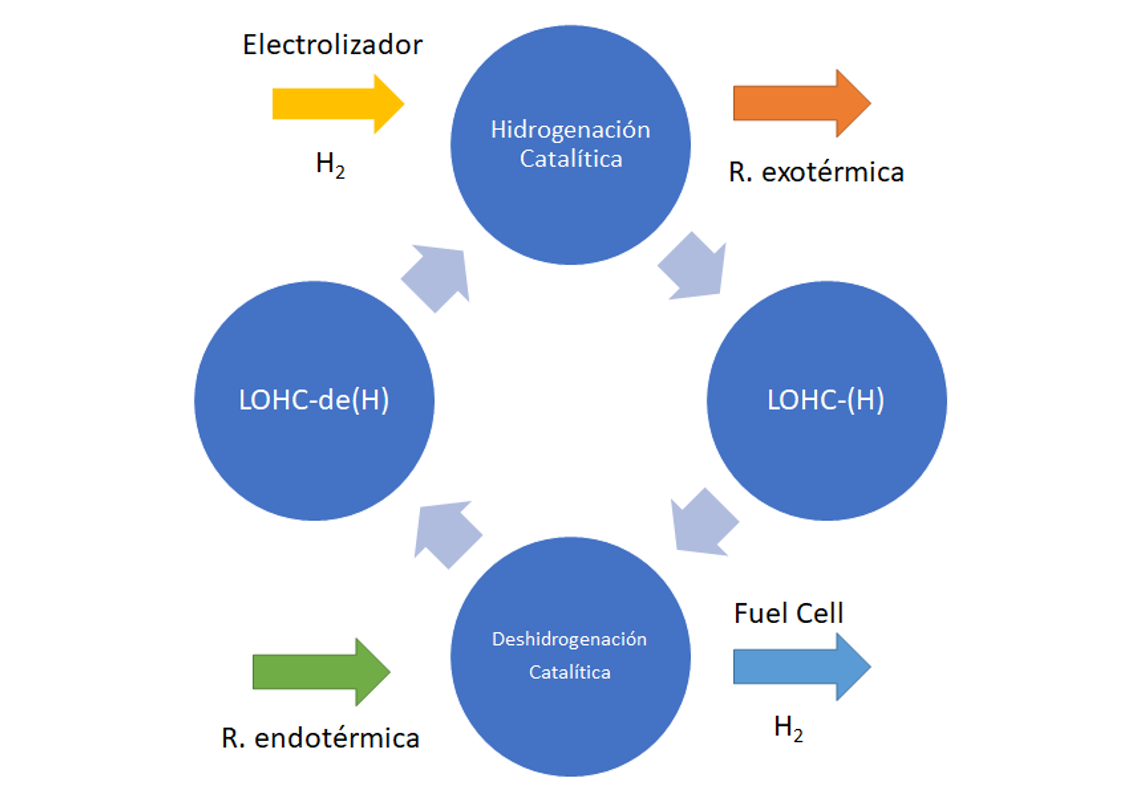
Figure 6. LOHC hydrogen storage process
Any unsaturated compound would be an example of a LOHC, since all of them can absorb hydrogen during hydrogenation. It is advantageous that both forms of LOHCs have a high boiling point in order to ease the separation of the hydrogen produced during dehydrogenation and a low freezing point to avoid clogging of equipment and to maintain the ability to pump at low temperatures. Although there are a large number of theoretically feasible options, further efforts are still needed to improve economic viability, safe handling and the amount of hydrogen carried within the liquid (estimated to be at least greater than 6% of weight).
Although methanol and formic acid are organic and liquid, they cannot be considered LOHCs according to this definition, because their dehydrogenation only leads to gaseous products.
Metal hydrides are composed of hydrogen molecules that dissociate into hydrogen molecules and are incorporated into the solid reticular structure of several metals and alloys such as magnesium, titanium, iron, manganese, nickel or chromium.
Heat is required to build up metal hydrides by absorption of hydrogen, which is distributed compactly throughout the hydride matrix. This reaction, which is reversible, is determined by the pressure of the hydrogen. When the pressure is above the balance pressure, the metal hydride is built up, whereas at a pressure below the balance point, hydrogen is released as the alloy reverts to its original status. This balance pressure is temperature-dependent since it increases by rising temperature and vice versa.
The hydrogen atoms are embedded in the metal structure, which results in higher volumetric storage densities than compressed and liquid hydrogen. This fact is shown in table 2, which shows the high volumetric density of metal hydrides such as LaNi₅, FeTi, MgH₂ and LiH.
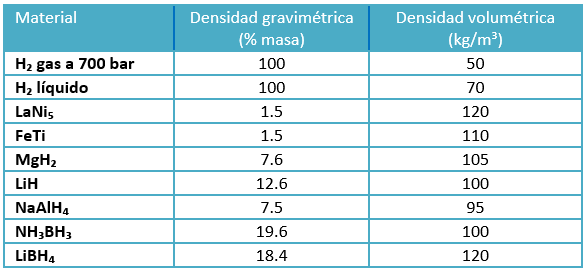
Table 2. Densities of different storage methods
Most metallic elements can make binary compounds containing hydrogen, so-called elemental hydrides. Nevertheless, most of them are unsuitable for hydrogen storage for several reasons. The main one is their low gravimetric density, Table 2. In addition, hydrogen is chemically bonded to metal hydrides, being these bonds much stronger than the physical bonds involved in hydrogen adsorption. This involves a higher energy required to release the chemically bonded hydrogen. Moreover, these hydrides are also susceptible to compounds such as oxygen and carbon monoxide, which decrease the hydrogen adsorption capacity.
In metal complex hydrides, the hydrogen is part of a complex anion, which is itself bonded to a metal cation. The main group of complex hydrides that are relevant for hydrogen storage are the alanates, the borohydrides and the amides. They are mainly composed of fairly light elements, which allows complex metal hydrides to have a quite high gravimetric hydrogen storage capacity, which has raised a lot of interest for using these materials in FCV solutions. The gravimetric and volumetric densities of the complex hydrides NaAlH₄, NH₃BH₃ and LiBH₄ can be seen in table 2.
Unfortunately, most complex hydrides require very high temperatures for dehydrogenation by thermolysis, and few can be reversibly dehydrogenated, usually requiring the presence of suitable catalysts or additives.
Chemical hydrides are compounds made up of a non-metallic element and hydrogen, joined by covalent bonds. They are lighter than metal hydrides and, contrary to metal hydrides, they are usually liquid under atmospheric conditions, simplifying their transport and storage as well as heat and mass transference at dehydrogenation and hydrogenation processes.
Among the chemical hydrides proposed to store hydrogen, many of them, such as methanol, ammonia and formic acid, are synthesised from natural gas and are nowadays used as feedstocks in the chemical industry. Hence, the usefulness of these chemicals goes beyond hydrogen storage. Widespread production of these chemicals is an advantage since much of the infrastructure necessary for their production, storage and distribution already exists. Moreover, the production of these chemicals in mass quantities using hydrogen obtained from the electrolysis of water rather than from natural gas refining, not only serves the purpose of storing hydrogen, but is also a way to reduce the use of fossil fuels during the production of these chemicals.
Conclusions
At this point it is clear that there are a wide range of solutions to store hydrogen at high densities. However, many of them are still being investigated, since there are still many challenges to be addressed.
What will be the most efficient or preferred way to transport and store hydrogen? It will take time to find out for sure, and will largely depend on its end use. For sure, renewable hydrogen will be a key part within the mix of storage technologies (hydrogen, batteries, etc.) which will allow the storage of surplus renewable energy for further consumption.
REFERENCES