Uses of electrolytic oxygen
Today, oxygen is mostly produced by cryogenic air distillation (CDA). In other words, the air is filtered, compressed, and liquefied and then separated into its various components (nitrogen, oxygen and argon) thanks to their different boiling points. Another alternative are systems based on PSA (Pressure Swing Adsorbers) in which the air is circulated through filters that separate the oxygen from the other components of the air.
The exclusive production of oxygen from electrolysis has not been considered until now, due to its high energy requirements since it costs less to extract oxygen from the air through the separation of its components than from the water molecule in its two elements (hydrogen and oxygen).
According to the plans promoted by the European Union, a considerable increase in the installed power and size of electrolysers in the member countries is expected. For this reason, it is interesting to consider a shared assessment of the products of the electrochemical reaction of water, hydrogen, and oxygen. This approach involves taking advantage of a “waste” product such as electrolytic oxygen and obtaining a revenue from the product, thus achieving a higher economic return on the use of electrolysers and, therefore, of renewable hydrogen.
The demand for industrial gases, including oxygen, is expected to increase in the coming years from 1.67 billion tons in 2023 to 2.07 billion tons by 2030. This increase in demand can be covered, to a certain extent, by electrolytic oxygen which, although it has a higher energy consumption, will be powered by renewable energies, reducing the carbon footprint of the final product.
Considering the possible use of electrolytic oxygen in the coming years, the possible uses of oxygen in industry must be properly studied.
Improved combustion efficiency in furnaces
The metallurgical, cement and glass industries have very high temperature thermal requirements to carry out their activities. High temperatures are achieved through the combustion of fossil fuels such as natural gas or diesel.
Combustion is a chemical reaction between a fuel, the previously mentioned, and a comburent. Oxygen contained in air is generally used as a comburent in industrial processes. The problem lies in the fact that air is composed, roughly speaking, of 79% nitrogen and 21% oxygen. Nitrogen has neither flammable nor combustible properties, being a thermally useless gas. Nitrogen also oxidises at high temperatures, generating NOX and consuming large amounts of energy in the process.
To avoid unnecessary expenditure of energy in the oxidation of nitrogen, we can replace the flow of combustion gas composed of air with a flow of pure oxygen. This substitution of the composition of the comburent gas is called oxy-combustion and, by avoiding the energy expense in heating nitrogen, we considerably increase the efficiency of the reaction. This results in lower fuel consumption within the reaction, which leads to a reduction of greenhouse gases and preservation of natural resources. On a technical level, oxycombustion makes it possible to improve control of heat transfer, which in return has an impact on fuel consumption and thus on greenhouse gas emissions.
Medical field
Oxygen is an essential element for the organism since it is in charge of energy production in the body and the elimination of toxins. Medicinal oxygen is a colorless, odorless and tasteless gas and has a minimum purity of 99.5%. Among the medicinal gases, oxygen is the most widely used as it has proven its importance in many modern medical practices and in numerous therapies.
This high purity oxygen can be administered in various ways and at multiple pressures depending on whether it is post-anesthesia applications, through respirators or by inhalation of medication by nebulisation.

Figure 1. Medicinal Oxygen Installation
Waste purification for biofuel production
Livestock and agro-industrial waste, sludge from urban wastewater treatment plants and the organic fraction of household waste are used as raw materials to produce biofuels, especially biogas.
Biogas is a biofuel composed mainly of methane (CH4) and carbon dioxide (CO2) that is generated from the fermentation or decomposition of organic matter, generating biogas with characteristics and uses similar to natural gas.
In more detail, the biogas is produced inside an anaerobic digester that is completely sealed to prevent the entry of light and air. Inside the digester there is a specific species of microorganisms which, in the absence of oxygen, digest the organic substances and generate the so-called biogas as a product. The biological reaction of the microorganisms also generates other products, such as water vapor and hydrogen sulfide (H2S), being this last one a highly corrosive compound.
Although the biological reaction takes place in the absence of oxygen, oxygen becomes more important in the treatment of the reaction products, in particular for the elimination of hydrogen sulfide by a process called oxygen dosing or micro-oxygenation. This process is accomplished by introducing a flow of oxygen into the upper part of the digester where it oxidises the hydrogen sulfide to elemental sulfur. Oxygen dosing also improves the quality of the digested sludge, reducing its foaming potential and accelerating the sludge drying process.
Purification of sewage water in wastewater treatment plants
Oxygen plays a very important role in water treatment, as the amount of oxygen present is one of the most crucial parameters for the successful completion of the process. The amount of dissolved oxygen in water that is accessible for consumption by aquatic microorganisms is called dissolved oxygen (DO).
The presence of these living organisms is key in the wastewater treatment process, as they are responsible for breaking down the organic matter present. In wastewater treatment plants, the amount of dissolved oxygen must be controlled exhaustively since an insufficient level of DO can inhibit biological activity and a large amount can also result in a reduction in the presence of organisms, since not all of them are able to survive under oxygen saturated conditions. Due to the need for this control, it is necessary to have oxygen storage to inject oxygen into the water to control the amount of microorganisms present.
Because of this need for oxygen, the implementation of electrolysers in water treatment plants may be of interest, since the treated water can be used by the electrolyser to produce hydrogen and oxygen, which in turn would be used in the aeration of the biological reactor. The heat generated by the electrolyser could also be used to heat the biological reactors, thus increasing the efficiency of the process.
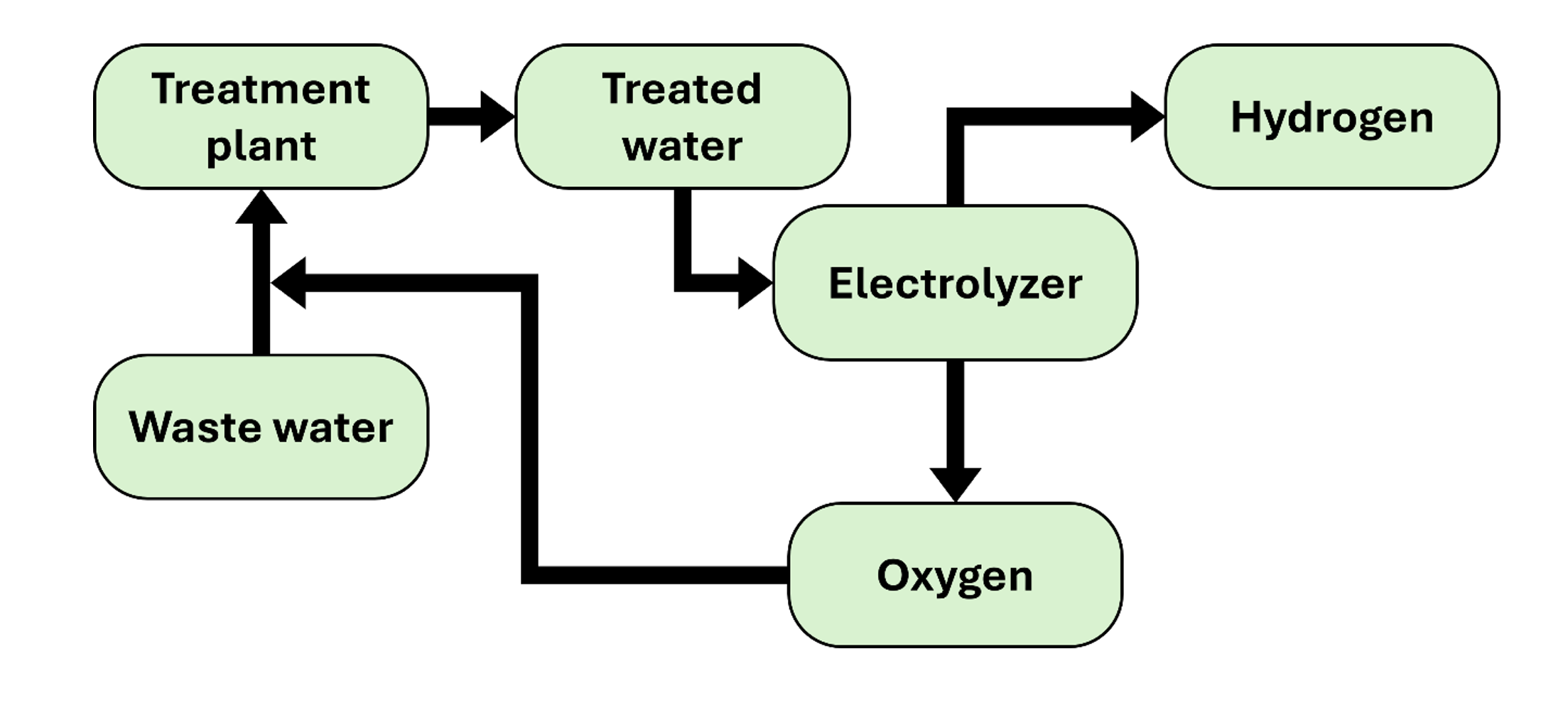
Figure 2. Schematic diagram of the Purifier-Electrolyser installation
Food industry
In the food industry, oxygen has several applications and uses, although it is not commonly used directly for food, it plays a fundamental role in multiple processes related to food treatment. Among the different processes we can highlight:
- Oxidation and food preservation: oxygen is used to maintain the respiration of food and prolong its lifespan. This helps to prevent spoilage and decomposition, maintaining the freshness and color of the products.
- Meat coloration: oxygen favors the reddish pigmentation of meats, making them look more attractive and avoiding meat wastage.
- Control of anaerobic microorganisms: the presence of oxygen eliminates possible anaerobic living organisms that may be present in the food, since they are not capable of surviving in this type of conditions.
- Fermentation in oenology and brewing: the presence of oxygen in fermentation processes enhances product quality.
Conclusions
If the EU targets for electrolyser power are met, there will be a high production of hydrogen and oxygen that should be exploited to avoid losing a highly versatile product, as mentioned in this article, thus promoting a circular economy of another part of the renewable hydrogen generation process and positively benefiting the economic efficiency of the electrolytic process, making it possible to lower the price of renewable hydrogen. Currently, oxygen is generated by cryogenic distillation of air, which is an energy-intensive method. If we add the production of oxygen by electrolysis, this will mean a considerable increase in the supply of the gas in the future, bringing down prices and favoring certain industrial uses hitherto set aside due to their high costs, such as oxy-combustion, making certain sectors that are difficult to decarbonise more efficient and reducing the carbon footprint considerably.
It is true that for many of the uses we have mentioned, electrolytic oxygen could not be used as it leaves the electrolyser, since depending on the type of technology used and the manufacturer, it will have a series of specific properties (flow rate, pressure, moisture content). Although it does not seem that the electrolytic oxygen purification technology is very complex, it could even be simpler than that associated with ASU systems, since these present problems due to the close boiling temperatures between the different elements that make up the air.
REFERENCES