Safety within hydrogen industry
Renewable hydrogen is already a reality in Spain. In the last few months we witnessed how hydrogen-powered buses are taking the streets of different cities in our country. Zaragoza has carried out a 10-day test on the airport line, in which 972 passengers were carried on board. Industry is also starting to bet on green hydrogen generation: Puertollano recently welcomed the arrival of the first tanks to store hydrogen for industrial processes.
As hydrogen is arriving at our daily lives to stay, let’s talk about its safety. Is hydrogen more dangerous than other fuels such as natural gas or diesel?
First of all, it is worth remembering that hydrogen did not emerge in the last 30 years, since it was discovered in 1766 by Henry Cavendish and it has been used in the chemical industry over the last century to produce fertilisers and refine petroleum. This is a guarantee: a lot of experience on handling this gas is available.
Renewable hydrogen is not only postulated as a substitute for the grey hydrogen that is already used today (500,000 tonnes of hydrogen are produced in Spain by methane refining) to reduce its associated emissions, but it is also destined to be used in other sectors such as mobility.
In another of our articles (Route of a H2 molecule from an HRS to the stack of an FCEV) we talked about the route that a molecule takes from the tank of a hydrogen plant (HRS) to the fuel cell. Today we are going to focus on why this journey is as safe as a ride on Dragon Khan.
Safety for plant design
Hydrogen is a colourless, odourless and tasteless gas, therefore safety is a crucial part for facilities that work using this element. All the components of a HRS installed in Spain have their individual CE marking and, before being installed, they have completed a factory test (FAT). After its installation, another test is carried out on site by an official entity to certify the normal operation of the HRS installation (SAT).
The design of the installation is aimed at avoiding the formation of Explosive Atmospheres (ATEX) that could cause any incidents. Hydrogen is the lightest gas which exists (its density is 89 g/m3 compared to the 1,293 g/m3 of air at normal conditions). Its high viscosity causes high leakage speeds if it leaks through an orifice. Thankfully, its dispersion in air is also high, allowing it to be diluted in a well-ventilated space. Good dimensioning of ATEX classified areas and proper safety measures minimise the hazards.
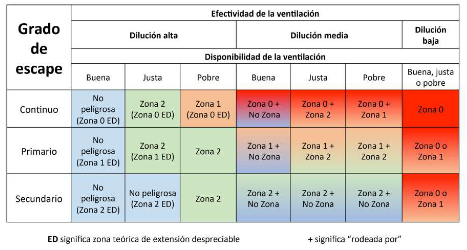
The best way to avoid risks is, without doubt, to avoid having Type 0 and 1 ATEX zones on site, as well as to ensure that ventilation is rated as good and to prevent simultaneous leaks. Even so, ATEX zones are not uncommon and remote, as it is more than likely that the reader will often visit a Type 1 ATEX zone while refuelling his car with petrol or diesel fuel, surprising though it may be.
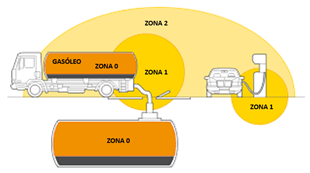
However, we can be confident that all hydrogen production and storage facilities must always be in compliance with national regulations regarding pressure devices, chemical production, gas storage, etc., as well as being installed by accredited installers, which minimises risks.
The European Commission, based on its commitment to hydrogen, is making important advances in terms of safety legislation and regulation. The European Hydrogen Safety Panel (EHSP) is one of the organisations entrusted with defining common guidelines for the appropriate use and handling of this element within the countries of the Union, developing templates, guides and studies on the matter.
Safety in the event of an accident
Moving forward: what if there is a leak? This is the question that every person in charge of designing a hydrogen facility asks himself. Surely, the Hindenburg Zeppelin will come to mind for many of us. Thankfully, we are not in 1937 and the HAZOP (Hazard and operatibilty study) and other safety elements can foresee any casuistry.
Hydrogen is a flammable gas and deflagration or detonation may occur in case of leakage when it reacts with air. Hydrogen is stored at high pressure and if leaking from an orifice will expand as the pressure drops. Hydrogen, among helium and neon, has a negative Joule Thomson coefficient, which causes it to heat up when it expands from high to low pressure. However, this is NOT the primary cause of it possibly igniting, as this phenomenon does not bring it to its self-ignition temperature (585°C).
Therefore, why can hydrogen ignite in the event of a leak? Hydrogen will jet out at subsonic and supersonic speeds, in accordance with different parameters such as pressure, temperature and the geometry of the leak. The high range of its combustibility threshold and the richness of oxygen within the air may cause it to ignite. However, technicians will already anticipate this and they will have estimated and modelled the length and shape of the leaked gas and therefore delimited the hazardous areas and focused the evacuation routes of the overpressure valves to safety areas far away from other equipment or in transit areas, ensuring the safety of equipment and people.
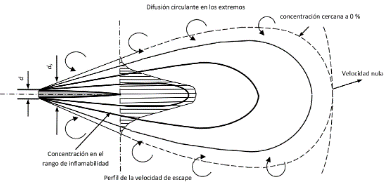
Finally, the million-dollar question: is hydrogen more dangerous than other fuels? This question was asked by Ulster researchers at the HySAFER centre, so they set up an experiment that led them to the following results, to which they answered in a very graphic way:
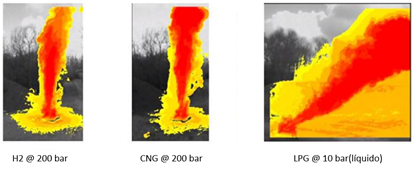

It is a remarkably interesting experiment, let us see why. The above images are thermographic images in order to measure the thermal radiation of the different flames (hydrogen at 200 bar versus CNG (compressed natural gas) at 200 bar and propane at 10 bar). These images show that propane has a very significant thermal footprint compared to hydrogen and CNG also has a higher thermal footprint than hydrogen. Notice that the images below are edited: the hydrogen flame is barely visible! When hydrogen is combusted (0.5 O2 + 2H+ + 2e– ↔ H2O), carbon generation is non-existent, which results in colourless and low radiant flames even if they are quite large.
Some safety elements for the successful operation of hydrogen facilities
Such extreme situations do not have to take place. There is a lot of work done to minimise the chances of such events happening. For their proper function, all hydrogen facilities have safety elements and protocols that help to react to unexpected situations and protect people and assets. As with other industries, redundant security measures are used to maximise protection as much as possible.
Breakaway
This is a device on the hose that prevents complete hydrogen leakage into the atmosphere in case a user forgets the hose connected to the vehicle’s receptacle and starts the vehicle, “taking the hose with him”. It is an automatic safety shut-off valve.
Pressure and temperature relief valves
These valves have pressure and temperature sensors designed to evacuate the hydrogen to the atmosphere in order to avoid overheating or overpressure that could damage the different elements of the installation, resulting in ruptures.
Inerting the installation
This manoeuvre is widespread in many industries, and hence its application in terms of prevention. Any oxygen that may have remained in the pipes and tanks is displaced by means of an inert gas, generally nitrogen, before the hydrogen is inserted into any part of the installation. The aim is to prevent the mixture of oxygen and hydrogen from reaching concentrations that could cause an explosion.
This protocol is also activated automatically in the event of leaks, thereby limiting concentrations and preventing explosive atmospheres. For this purpose, not only hydrogen detectors but also oxygen detectors are used to check both gas concentrations.
To sum up, hydrogen is no more dangerous than other fuel products commonly used, and even in many situations, it is safer than CNG or LPGs. The knowledge of hydrogen properties, appropriate technology and safety protocols make hydrogen a safe energy vector.
At SynerHy, our job is to advise and train companies so they can understand how hydrogen behaves and how to work with it, thus making it the definitive energy solution.
In collaboration with Juan Palencia, Álvaro Reyes and Yago Reglero.
REFERENCES
Fundamentals of Hydrogen Safety Engineering – Molkow